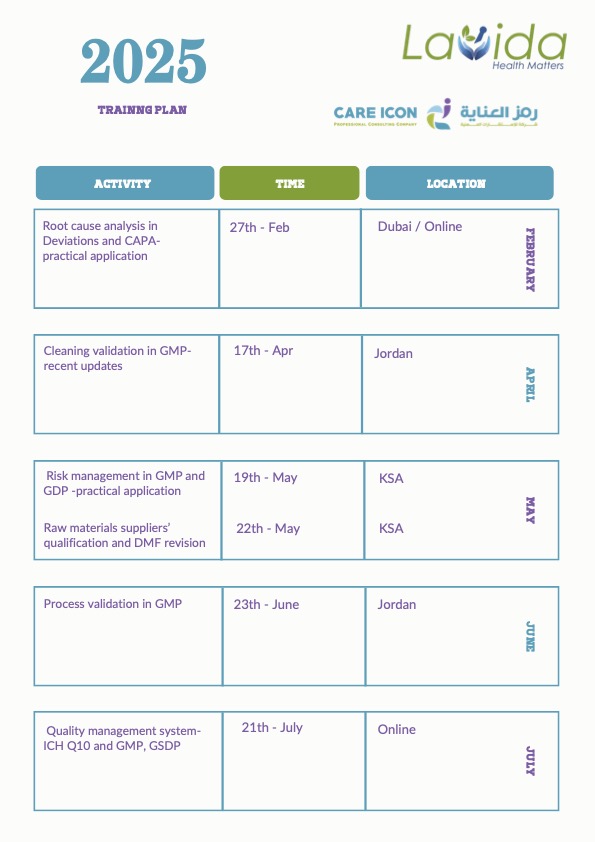

2025 Training Plan: Enhance Your Expertise in GMP, GDP, and Quality Management
The 2025 Training Plan is designed to provide professionals with the latest knowledge and hands-on expertise in Good Manufacturing Practices (GMP), Good Distribution Practices (GDP), quality management, risk assessment, and regulatory compliance.
With sessions available both online and in major locations such as Dubai, Jordan, and KSA, this program is a must for professionals in the pharmaceutical and financial sectors looking to expand their skillset.
📅 Key Training Sessions
February
•27th Feb: Root Cause Analysis in Deviations and CAPA – Practical Application
•Cleaning Validation in GMP – Recent Updates (Dubai / Online)
April
•17th Apr: Risk Management in GMP and GDP – Practical Application (Jordan)
May
•19th – 22nd May: Raw Materials Suppliers’ Qualification and DMF Revision (KSA)
June
•23rd June: Process Validation in GMP (Jordan)
July
•21st July: Quality Management System – ICH Q10 and GMP, GSDP (Online / KSA)
August
•25th Aug: Data Integrity in Pharmaceutical Manufacturing, Storage, and Distribution
•Good Laboratory Practices (Online)
September
•22nd Sep: Stores Design, Mapping Studies, and Equipment Qualification (Dubai)
October
•27th – 28th Oct: Good Documentation Practice (KSA)
November
•22nd Nov: External Auditing, Qualifying Service Providers, and Technical Agreements (Dubai)
•24th Nov: EU-GDP Standard Regulations and Accreditation to EU-GDP (KSA)
🎯 Why Attend?
✔ Gain insights into the latest regulations and best practices in GMP and GDP.
✔ Learn from industry experts with real-world experience.
✔ Boost your career opportunities by staying updated on compliance and quality management.
✔ Network with professionals and industry leaders from across the region.
📝 How to Register?
Secure your spot by completing the registration form here:
Don’t miss this opportunity to upgrade your skills and stay ahead in the industry. Register today! 🚀